Hear from people who are living with acquired/immune-mediated thrombotic thrombocytopenic purpura (aTTP/iTTP*)
Hear stories from real people. Follow them on their personal journeys from diagnosis to their day-to-day experiences of living with aTTP/iTTP. Each person’s experience is unique and individual results may vary. These individuals were compensated for their time creating these videos. Remember, your doctor is your best source of information. Be sure to ask your doctor any questions you may have.

James and Dr Masias: Educating others about aTTP/iTTP
“It’s the first time I’ve heard of anything specific for [aTTP/iTTP]. It lets me know that somebody is trying to work on this and trying to figure out how to help.”
James, real patient. Individual results may vary. James is being compensated by Sanofi.

Moving forward: Alex’s aTTP/iTTP experience
“On my second experience with [aTTP/iTTP], my new doctor mentioned that there was a medication that could be helpful for me called CABLIVI.”
Alex, real patient. Individual results may vary. Alex is being compensated by Sanofi.

Coping with relapse: Heather’s aTTP/iTTP story
“Even though I know a relapse is possible at any time, I’m confident in knowing that CABLIVI will be there.”
Heather, real patient. Individual results may vary. Heather is being compensated by Sanofi.
Learn more about CABLIVI
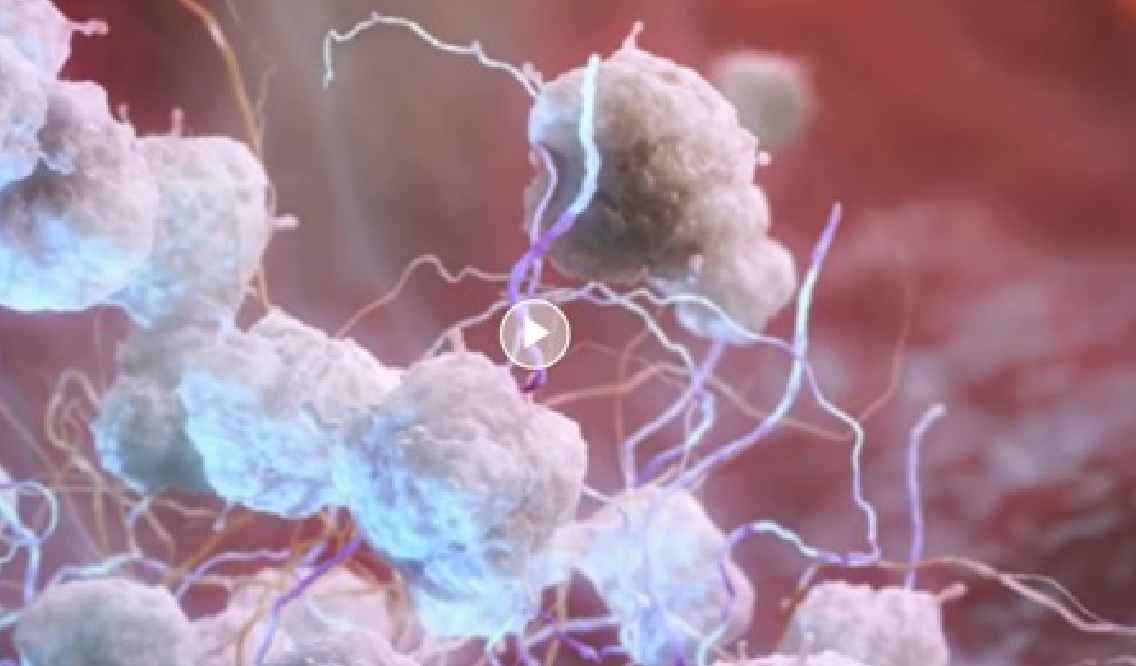
How CABLIVI works
When combined with PEX and immunosuppression, CABLIVI helps stop platelets from sticking to vWF and helps prevent your body from forming dangerous blood clots during an aTTP/iTTP episode.
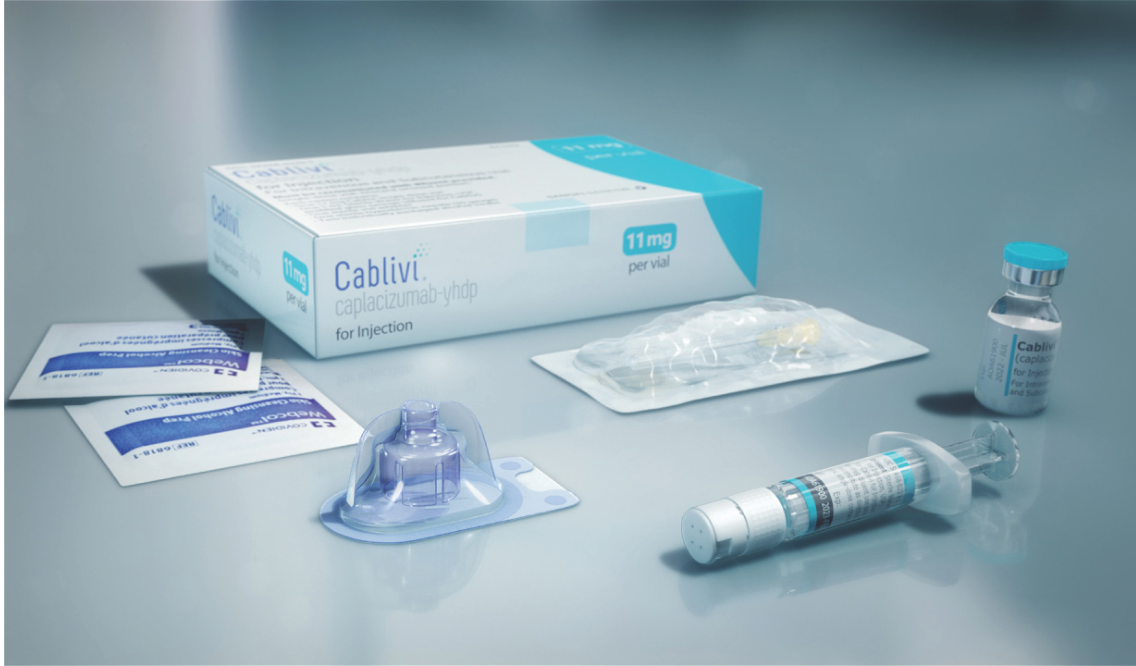
How to take CABLIVI
“Your healthcare provider should show you how to prepare and inject CABLIVI properly before your first injection.”
CABLIVI, in combination with PEX and immunosuppression, can help you take on aTTP/iTTP with confidence
Resources about CABLIVI are available, including how to take it at home and suggestions on what to discuss with doctors
*aTTP is also known as iTTP. You and your healthcare team can use either term.
aTTP/iTTP=acquired/immune-mediated thrombotic thrombocytopenic purpura; vWF=von Willebrand factor.
IMPORTANT SAFETY INFORMATION AND INDICATIONS
Who should not take CABLIVI?
Do not take CABLIVI if you’ve had an allergic reaction to caplacizumab-yhdp or to any of the ingredients in CABLIVI.
What should I tell my healthcare team before starting CABLIVI?
Tell your doctor if you have a medical condition including if you have a bleeding disorder. Tell your doctor about any medicines you take, including medicines that increase your risk of bleeding such as anti-coagulants and anti-platelet agents.
Talk to your doctor before scheduling any surgery, medical or dental procedure.
What are the possible side effects of CABLIVI?
CABLIVI can cause severe bleeding. In clinical studies, severe bleeding adverse reactions of nosebleed, bleeding from the gums, bleeding in the stomach or intestines, and bleeding from the uterus were each reported in 1% of subjects. In the post-marketing setting, cases of life-threatening and fatal bleeding were reported in patients receiving CABLIVI. Contact your doctor immediately if symptoms of excessive bruising, excessive bleeding, or major bleeding occur. Signs and symptoms of bleeding include: pain, swelling or discomfort, prolonged bleeding from cuts, increased menstrual flow or vaginal bleeding, nosebleeds, bleeding of gums from brushing, unusual bleeding or bruising, red or dark brown urine, red or tar black stools, headache, dizziness, or weakness.
You may have a higher risk of bleeding if you have a bleeding disorder (i.e. hemophilia) or if you take other medicines that increase your risk of bleeding such as anti-coagulants and anti-platelet agents.
CABLIVI should be stopped for 7 days before surgery or any medical or dental procedure. Talk to your doctor before you stop taking CABLIVI.
The most common side effects include nosebleed, headache and bleeding gums.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of CABLIVI. Call your doctor for medical advice about side effects.
What is CABLIVI?
CABLIVI (caplacizumab-yhdp) is a prescription medicine used for the treatment of adults with acquired thrombotic thrombocytopenic purpura (aTTP), in combination with plasma exchange and immunosuppressive therapy.
Please see full Prescribing Information.
Instructions For Use
Sharps Medical Waste Disposal (PDF)
Learn more about Sanofi’s commitment to fighting counterfeit drugs.
IMPORTANT SAFETY INFORMATION AND INDICATIONS
Who should not take CABLIVI?
Do not take CABLIVI if you’ve had an allergic reaction to caplacizumab-yhdp or to any of the ingredients in CABLIVI.
What should I tell my healthcare team before starting CABLIVI?
Tell your doctor if you have a medical condition including if you have a bleeding disorder. Tell your doctor about any medicines you take, including medicines that increase your risk of bleeding such as anti-coagulants and anti-platelet agents.
Talk to your doctor before scheduling any surgery, medical or dental procedure.
What are the possible side effects of CABLIVI?
CABLIVI can cause severe bleeding. In clinical studies, severe bleeding adverse reactions of nosebleed, bleeding from the gums, bleeding in the stomach or intestines, and bleeding from the uterus were each reported in 1% of subjects. In the post-marketing setting, cases of life-threatening and fatal bleeding were reported in patients receiving CABLIVI. Contact your doctor immediately if symptoms of excessive bruising, excessive bleeding, or major bleeding occur. Signs and symptoms of bleeding include: pain, swelling or discomfort, prolonged bleeding from cuts, increased menstrual flow or vaginal bleeding, nosebleeds, bleeding of gums from brushing, unusual bleeding or bruising, red or dark brown urine, red or tar black stools, headache, dizziness, or weakness.
You may have a higher risk of bleeding if you have a bleeding disorder (i.e. hemophilia) or if you take other medicines that increase your risk of bleeding such as anti-coagulants and anti-platelet agents.
CABLIVI should be stopped for 7 days before surgery or any medical or dental procedure. Talk to your doctor before you stop taking CABLIVI.
The most common side effects include nosebleed, headache and bleeding gums.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of CABLIVI. Call your doctor for medical advice about side effects.
What is CABLIVI?
CABLIVI (caplacizumab-yhdp) is a prescription medicine used for the treatment of adults with acquired thrombotic thrombocytopenic purpura (aTTP), in combination with plasma exchange and immunosuppressive therapy.
Please see full Prescribing Information.
Instructions For Use
Sharps Medical Waste Disposal (PDF)
Learn more about Sanofi’s commitment to fighting counterfeit drugs.
IMPORTANT SAFETY INFORMATION AND INDICATIONS
Who should not take CABLIVI?
Do not take CABLIVI if you’ve had an allergic reaction to caplacizumab-yhdp or to any of the ingredients in CABLIVI.
What should I tell my healthcare team before starting CABLIVI?
Tell your doctor if you have a medical condition including if you have a bleeding disorder. Tell your doctor about any medicines you take, including medicines that increase your risk of bleeding such as anti-coagulants and anti-platelet agents.
Talk to your doctor before scheduling any surgery, medical or dental procedure.
What are the possible side effects of CABLIVI?
CABLIVI can cause severe bleeding. In clinical studies, severe bleeding adverse reactions of nosebleed, bleeding from the gums, bleeding in the stomach or intestines, and bleeding from the uterus were each reported in 1% of subjects. In the post-marketing setting, cases of life-threatening and fatal bleeding were reported in patients receiving CABLIVI. Contact your doctor immediately if symptoms of excessive bruising, excessive bleeding, or major bleeding occur. Signs and symptoms of bleeding include: pain, swelling or discomfort, prolonged bleeding from cuts, increased menstrual flow or vaginal bleeding, nosebleeds, bleeding of gums from brushing, unusual bleeding or bruising, red or dark brown urine, red or tar black stools, headache, dizziness, or weakness.
You may have a higher risk of bleeding if you have a bleeding disorder (i.e. hemophilia) or if you take other medicines that increase your risk of bleeding such as anti-coagulants and anti-platelet agents.
CABLIVI should be stopped for 7 days before surgery or any medical or dental procedure. Talk to your doctor before you stop taking CABLIVI.
The most common side effects include nosebleed, headache and bleeding gums.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of CABLIVI. Call your doctor for medical advice about side effects.
What is CABLIVI?
CABLIVI (caplacizumab-yhdp) is a prescription medicine used for the treatment of adults with acquired thrombotic thrombocytopenic purpura (aTTP), in combination with plasma exchange and immunosuppressive therapy.
Please see full Prescribing Information.
Instructions For Use
Sharps Medical Waste Disposal (PDF)
Learn more about Sanofi’s commitment to fighting counterfeit drugs.


