CABLIVI is the first and only FDA-approved therapy for adults and pediatric patients 12 years and older with aTTP/iTTP* designed to work with plasma exchange (PEX) and immunosuppressive therapy
CABLIVI was tested in a clinical study of 145 adults with aTTP/iTTP
72
72 people were given
CABLIVI + PEX +
immunosuppressive therapy
vs
73
73 people were given a placebo
(an injection without any medicine) +
PEX + immunosuppressive therapy
After treatment was stopped, everyone was followed by their doctor for 28 more days.
CABLIVI CONFIDENCE—In the clinical study, the CABLIVI difference was clear
CABLIVI helped return platelet counts to normal significantly faster when added to PEX and immunosuppressive therapy.
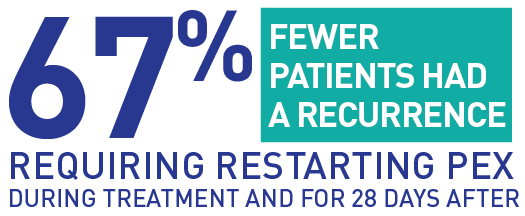
67% fewer patients in the CABLIVI group had a recurrence, where a drop in platelets required restarting PEX, during treatment and for 28 days after.
Significantly fewer people had another episode of aTTP/iTTP that required starting PEX again within the full study period (while taking CABLIVI and 28 days after stopping CABLIVI) in the CABLIVI group vs the placebo group: 13% receiving CABLIVI (9 people) vs 38% receiving placebo (28 people).
CABLIVI significantly lowered aTTP/iTTP events
74% reduction in serious aTTP/iTTP events for patients treated with CABLIVI.
CABLIVI significantly lowered the chance of serious problems linked to aTTP/iTTP during treatment. 12.7% (9 patients) treated with CABLIVI had an aTTP/iTTP-related event vs 49.3% (36 patients) treated with placebo.
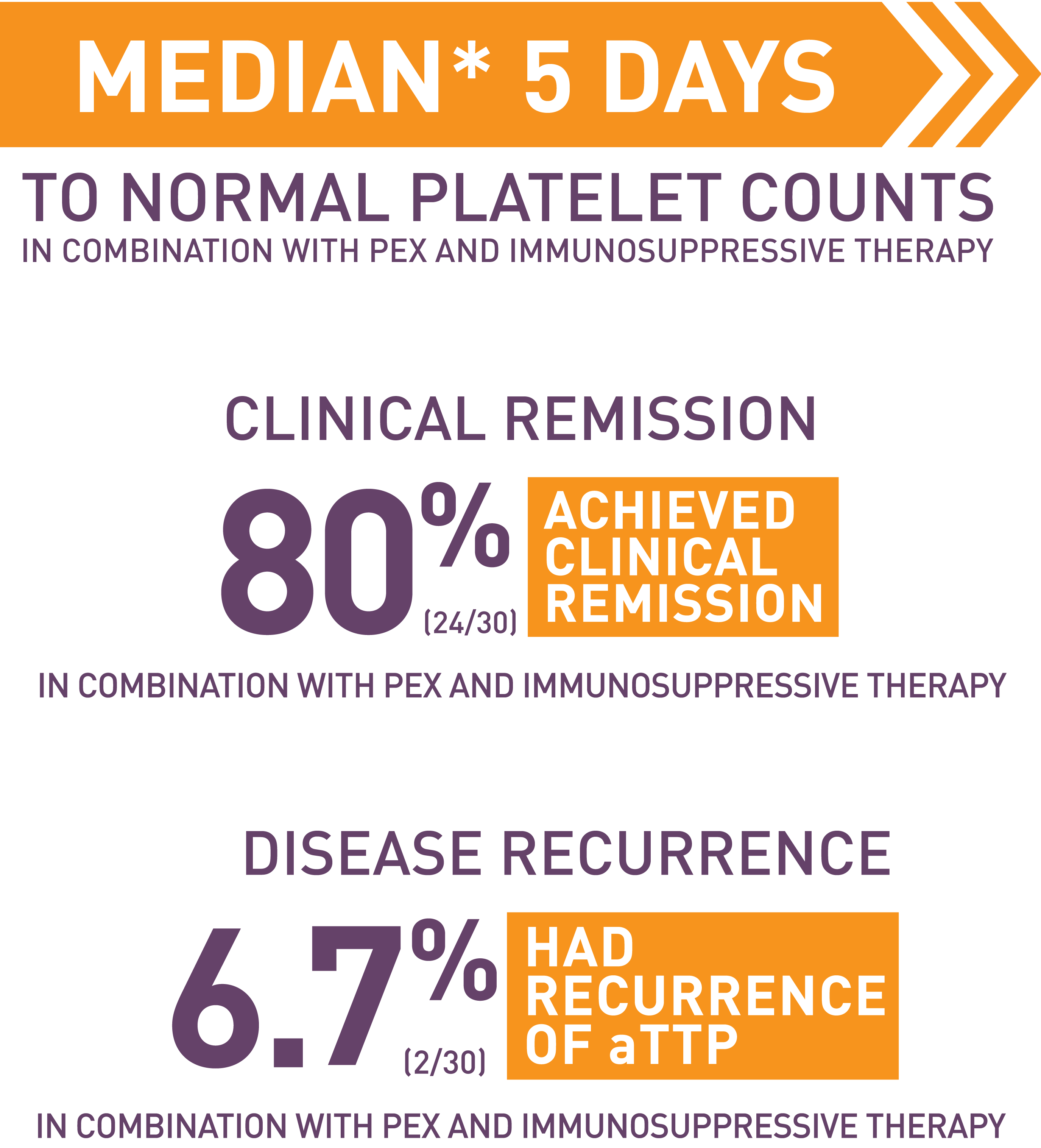
CABLIVI CONFIDENCE IN PEDIATRIC PATIENTS 12 YEARS OF AGE AND OLDER
The use of CABLIVI in combination with PEX and immunosuppressive therapy was evaluated in pediatric patients 12 years of age and older with aTTP/iTTP
Researchers evaluated data from an observational, retrospective chart review to see how safe and effective CABLIVI was in 30 younger patients with aTTP ranging in age from 2 to 18. In the study, most patients (27/30) began CABLIVI at the adult dose. The safety and effectiveness of CABLIVI in pediatric patients younger than 12 years of age have not been established.
CABLIVI is effective in pediatric patients with aTTP/iTTP
*Median=the middle value in a dataset, where half of the results are above it and half are below it.
The International Society on Thrombosis and Haemostasis (ISTH) Guidelines Updated: More Evidence Supports CABLIVI
The ISTH, a recognized global authority in the treatment and management of hematology, recommends using CABLIVI, PEX, and immunosuppression for adults experiencing an aTTP/iTTP episode
-
The ISTH Guidelines help physicians make informed treatment decisions for people with aTTP/iTTP
-
The ISTH Guidelines suggest using CABLIVI with PEX and immunosuppression for adults experiencing their first episode or another recurrence
The ISTH Treatment Guidelines suggest using CABLIVI for adults experiencing their first episode or another aTTP/iTTP episode.
Like any medication, side effects are possible with CABLIVI
|
HemAssistTM Sanofi Support is dedicated to your needs, from starting CABLIVI in the hospital to continuing at home
|
*aTTP is also known as iTTP. You and your healthcare team can use either term.
†Recommendation based on laboratory testing and diagnosis.
aTTP/iTTP=acquired/immune-mediated thrombotic thrombocytopenic purpura; PEX=plasma exchange.
INDICATION AND IMPORTANT SAFETY INFORMATION
Who should not take CABLIVI?
Do not take CABLIVI if you’ve had an allergic reaction to caplacizumab-yhdp or to any of the ingredients in CABLIVI.
What should I tell my healthcare team before starting CABLIVI?
Tell your doctor if you have a medical condition including if you have a bleeding disorder. Tell your doctor about any medicines you take, including medicines that increase your risk of bleeding such as antiplatelets, thrombolytics, heparin, or anti-coagulants.
Talk to your doctor before scheduling any surgery, medical or dental procedure.
What are the possible side effects of CABLIVI?
CABLIVI can cause severe bleeding. In clinical studies, severe bleeding adverse reactions of nosebleed, bleeding from the gums, bleeding in the stomach or intestines, and bleeding from the uterus were each reported in 1% of subjects. In the post-marketing setting, cases of life-threatening and fatal bleeding were reported in patients receiving CABLIVI. Contact your doctor immediately if symptoms of excessive bruising, excessive bleeding, or major bleeding occur. Signs and symptoms of bleeding include: pain, swelling or discomfort, prolonged bleeding from cuts, increased menstrual flow or vaginal bleeding, nosebleeds, bleeding of gums from brushing, unusual bleeding or bruising, red or dark brown urine, red or tar black stools, headache, dizziness, or weakness.
You may have a higher risk of bleeding if you have a bleeding disorder (i.e. hemophilia) or if you take other medicines that increase your risk of bleeding such as anti-coagulants and anti-platelet agents.
CABLIVI should be stopped for 7 days before surgery or any medical or dental procedure. Talk to your doctor before you stop taking CABLIVI.
The most common side effects in adults include nosebleed, headache and bleeding gums.
In children, the most reported side effects include nosebleed and rapid heartbeat.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of CABLIVI. Call your doctor for medical advice about side effects.
What is CABLIVI?
CABLIVI (caplacizumab-yhdp) is a prescription medicine used for the treatment of adults and children 12 years and older with acquired thrombotic thrombocytopenic purpura (aTTP), in combination with plasma exchange and immunosuppressive therapy.
Please see full Prescribing Information.
Instructions For Use
Sharps Medical Waste Disposal (PDF)
Learn more about Sanofi’s commitment to fighting counterfeit drugs.
INDICATION AND IMPORTANT SAFETY INFORMATION
Who should not take CABLIVI?
Do not take CABLIVI if you’ve had an allergic reaction to caplacizumab-yhdp or to any of the ingredients in CABLIVI.
What should I tell my healthcare team before starting CABLIVI?
Tell your doctor if you have a medical condition including if you have a bleeding disorder. Tell your doctor about any medicines you take, including medicines that increase your risk of bleeding such as antiplatelets, thrombolytics, heparin, or anti-coagulants.
Talk to your doctor before scheduling any surgery, medical or dental procedure.
What are the possible side effects of CABLIVI?
CABLIVI can cause severe bleeding. In clinical studies, severe bleeding adverse reactions of nosebleed, bleeding from the gums, bleeding in the stomach or intestines, and bleeding from the uterus were each reported in 1% of subjects. In the post-marketing setting, cases of life-threatening and fatal bleeding were reported in patients receiving CABLIVI. Contact your doctor immediately if symptoms of excessive bruising, excessive bleeding, or major bleeding occur. Signs and symptoms of bleeding include: pain, swelling or discomfort, prolonged bleeding from cuts, increased menstrual flow or vaginal bleeding, nosebleeds, bleeding of gums from brushing, unusual bleeding or bruising, red or dark brown urine, red or tar black stools, headache, dizziness, or weakness.
You may have a higher risk of bleeding if you have a bleeding disorder (i.e. hemophilia) or if you take other medicines that increase your risk of bleeding such as anti-coagulants and anti-platelet agents.
CABLIVI should be stopped for 7 days before surgery or any medical or dental procedure. Talk to your doctor before you stop taking CABLIVI.
The most common side effects in adults include nosebleed, headache and bleeding gums.
In children, the most reported side effects include nosebleed and rapid heartbeat.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of CABLIVI. Call your doctor for medical advice about side effects.
What is CABLIVI?
CABLIVI (caplacizumab-yhdp) is a prescription medicine used for the treatment of adults and children 12 years and older with acquired thrombotic thrombocytopenic purpura (aTTP), in combination with plasma exchange and immunosuppressive therapy.
Please see full Prescribing Information.
Instructions For Use
Sharps Medical Waste Disposal (PDF)
Learn more about Sanofi’s commitment to fighting counterfeit drugs.
INDICATION AND IMPORTANT SAFETY INFORMATION
Who should not take CABLIVI?
Do not take CABLIVI if you’ve had an allergic reaction to caplacizumab-yhdp or to any of the ingredients in CABLIVI.
What should I tell my healthcare team before starting CABLIVI?
Tell your doctor if you have a medical condition including if you have a bleeding disorder. Tell your doctor about any medicines you take, including medicines that increase your risk of bleeding such as antiplatelets, thrombolytics, heparin, or anti-coagulants.
Talk to your doctor before scheduling any surgery, medical or dental procedure.
What are the possible side effects of CABLIVI?
CABLIVI can cause severe bleeding. In clinical studies, severe bleeding adverse reactions of nosebleed, bleeding from the gums, bleeding in the stomach or intestines, and bleeding from the uterus were each reported in 1% of subjects. In the post-marketing setting, cases of life-threatening and fatal bleeding were reported in patients receiving CABLIVI. Contact your doctor immediately if symptoms of excessive bruising, excessive bleeding, or major bleeding occur. Signs and symptoms of bleeding include: pain, swelling or discomfort, prolonged bleeding from cuts, increased menstrual flow or vaginal bleeding, nosebleeds, bleeding of gums from brushing, unusual bleeding or bruising, red or dark brown urine, red or tar black stools, headache, dizziness, or weakness.
You may have a higher risk of bleeding if you have a bleeding disorder (i.e. hemophilia) or if you take other medicines that increase your risk of bleeding such as anti-coagulants and anti-platelet agents.
CABLIVI should be stopped for 7 days before surgery or any medical or dental procedure. Talk to your doctor before you stop taking CABLIVI.
The most common side effects in adults include nosebleed, headache and bleeding gums.
In children, the most reported side effects include nosebleed and rapid heartbeat.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of CABLIVI. Call your doctor for medical advice about side effects.
What is CABLIVI?
CABLIVI (caplacizumab-yhdp) is a prescription medicine used for the treatment of adults and children 12 years and older with acquired thrombotic thrombocytopenic purpura (aTTP), in combination with plasma exchange and immunosuppressive therapy.
Please see full Prescribing Information.
Instructions For Use
Sharps Medical Waste Disposal (PDF)
Learn more about Sanofi’s commitment to fighting counterfeit drugs.







.png)