Acquired thrombotic thrombocytopenic purpura (aTTP) is a serious condition that can be managed if treated as soon as possible
aTTP is a rare, life-threatening blood disorder that causes small clots throughout the body. aTTP is also known as immune-mediated thrombotic thrombocytopenic purpura (iTTP). aTTP and iTTP are the same condition. Your healthcare team may use either term.
aTTP/iTTP is a lifelong condition. Some people may only experience 1 episode of aTTP/iTTP. However, some people have additional episodes, called “relapses,” over their lifetime.
aTTP/iTTP episodes are medical emergencies that can be sudden and unexpected
You may also hear the term TTP used by your care team. TTP includes both acquired/immune-mediated TTP and hereditary or congenital TTP. Hereditary or congenital TTP is a condition people are born with that is extremely rare. Not even 5% of cases are hereditary.
aTTP/iTTP can cause dangerous blood clots if not treated in a timely manner
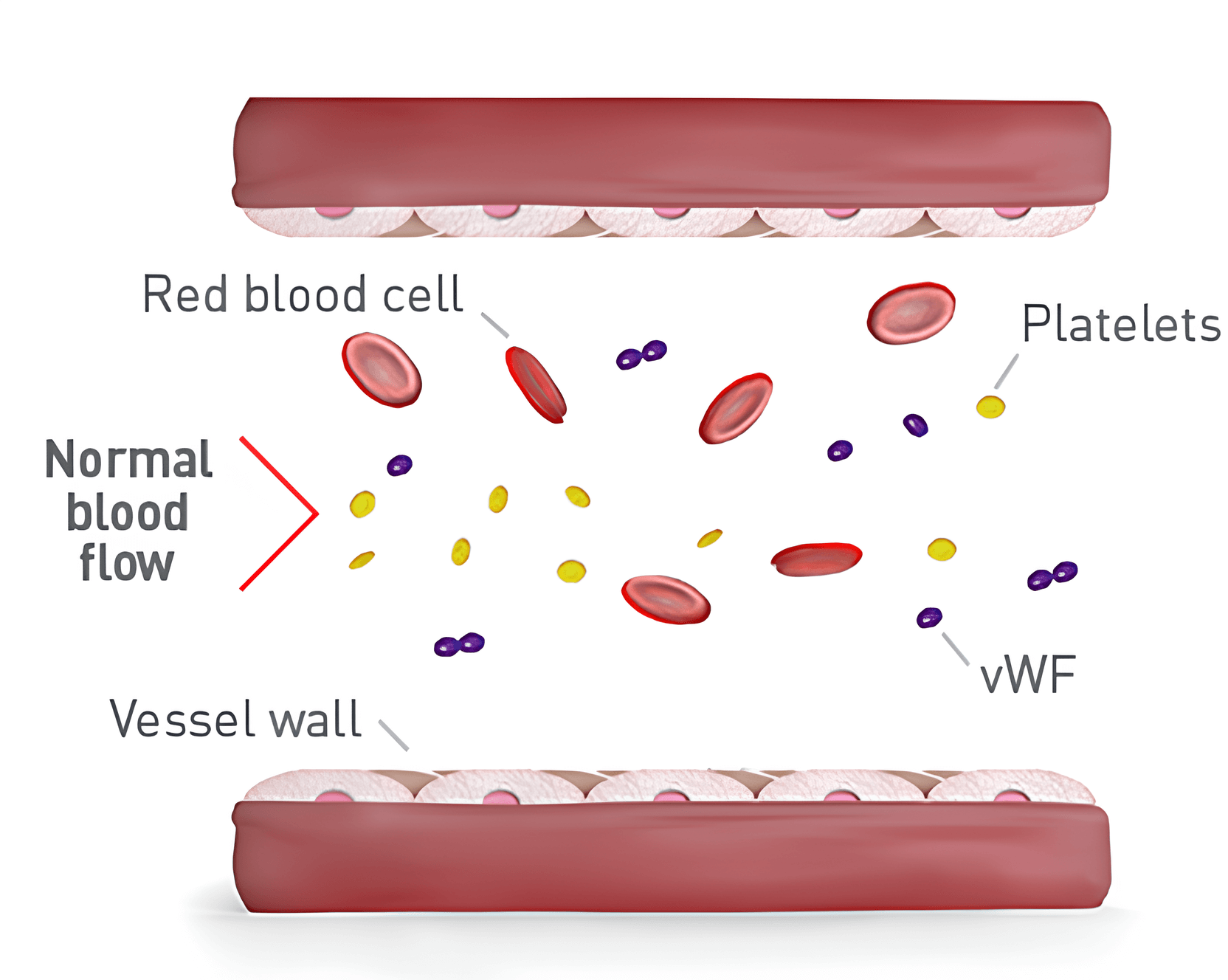
What happens in the blood normally
Blood vessels are tubes that carry blood through your body. A blood clot is a clump of small blood cells, called platelets, that are stuck together. Normally, platelets float in your blood and form clots only when you need them to help you stop bleeding.
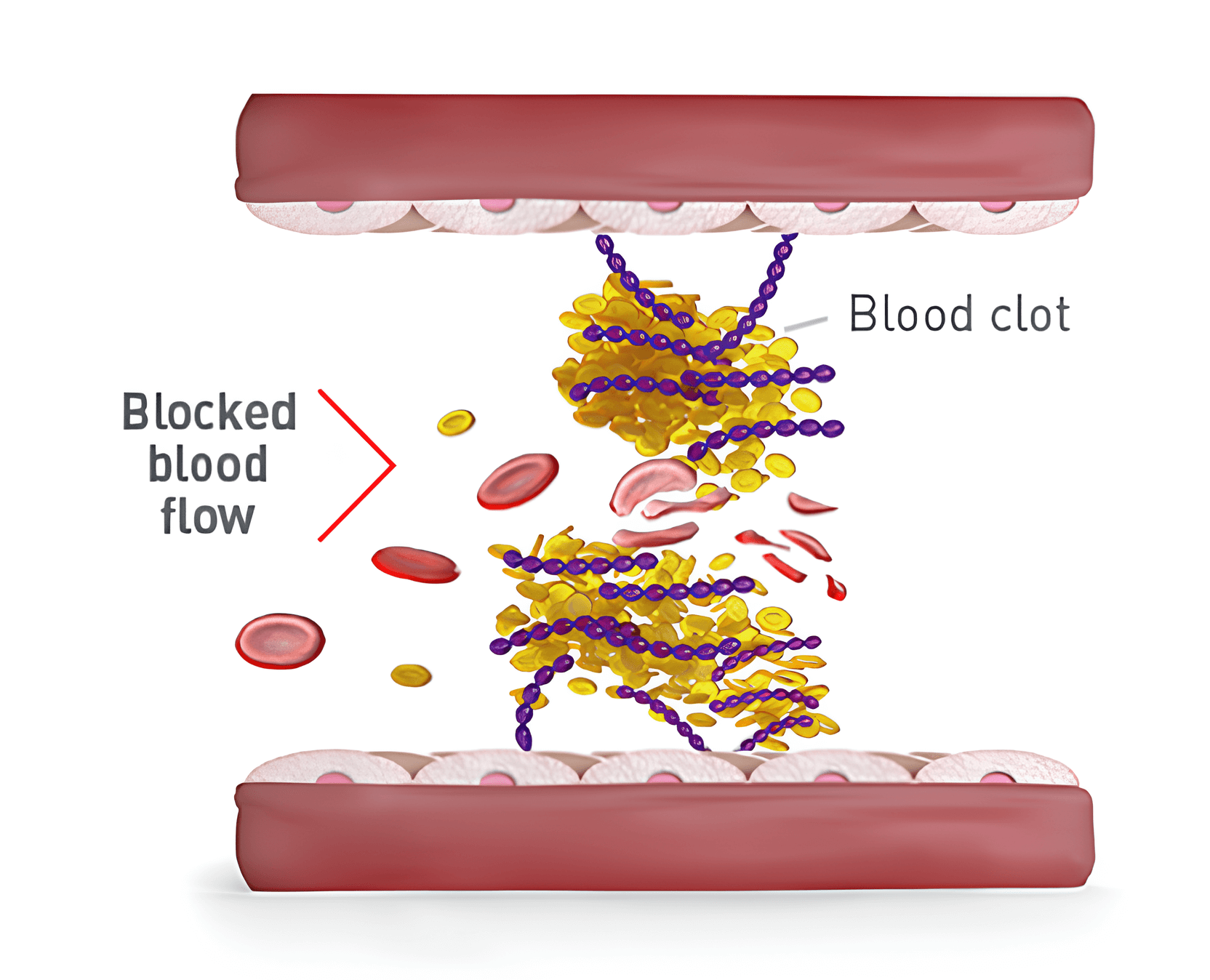
What happens in the blood with aTTP/iTTP
When you have aTTP/iTTP, platelets make clots when they’re not supposed to in a blood vessel. This makes it hard for blood to flow through the body to important organs like the heart, brain, and kidneys.
Blood clots in aTTP/iTTP can:
Block the flow of blood to important organs (brain, heart, or kidneys) that can lead to organ damage
Destroy red blood cells that carry oxygen through the body, so the body can’t get the oxygen it needs. This is called hemolytic anemia
To understand aTTP/iTTP further, you need to understand the roles of vWF and ADAMTS13
-
von Willebrand factor (vWF) is an important part of your blood. It is made of strands that attract platelets to make blood clots to help stop bleeding when you need them
-
ADAMTS13 is a protein in your blood. It keeps vWF strands from growing too long by cutting the strands into small pieces
-
Without ADAMTS13, the vWF strands grow too long, attracting a lot of platelets, and form clots you don’t need. These clots may become dangerous to your health
-
When you have aTTP/iTTP, the immune system attacks ADAMTS13
aTTP/iTTP causes 3 things to go wrong in the body

1
The immune system attacks ADAMTS13 with autoantibodies
What it means for the body
If ADAMTS13 is being attacked, there won’t be enough in the blood
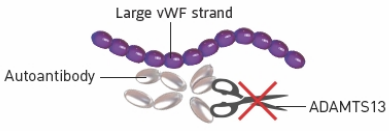
2
Because there is not enough ADAMTS13, vWF strands don’t get cut
What it means for the body
When vWF strands grow too long, they attract a lot of platelets when they are not supposed to, which means there are not enough platelets in the blood
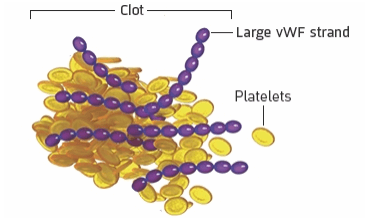
3
When long strands of vWF attract platelets, dangerous blood clots form in small blood vessels
What it means for the body
These blood clots can cause serious health problems like stroke, heart attack, seizures, and organ damage
Your doctor may perform certain lab tests to monitor your health at diagnosis and throughout an aTTP/iTTP episode
Platelet and red blood cell count
Your doctor will confirm if you have fewer platelets and red blood cells than you should and if they are improving with treatment
ADAMTS13 enzyme activity levels
Your doctor will see how well your ADAMTS13 is working. Low levels mean aTTP/iTTP is active. Low levels can still occur during treatment and after other symptoms improve
Organ function
Because clots can impact the organs, your doctor will perform different tests to check how well your organs are working
Who is affected by aTTP/iTTP?
It’s not fully understood why some people develop aTTP/iTTP—it can affect anyone.
<2000 people
aTTP/iTTP affects fewer than 2000 people in the United States every year.
30 to 50 years old
aTTP/iTTP usually affects people 30 to 50 years of age, but it can affect people of any age.
Women
aTTP/iTTP is more common in women than men.
Black ethnicity
aTTP/iTTP is more likely to affect people of Black ethnicity.
Learn more about aTTP/iTTP from someone living with it and someone treating it
James & Dr. Masias: Educating Others About aTTP
Playing this video will share cookies with YouTube.
CABLIVI (caplacizumab-yhdp) is indicated for the treatment of adult patients with acquired Thrombotic Thrombocytopenic Purpura (aTTP), in combination with plasma exchange and immunosuppressive therapy.
Please stay tuned for the Important Safety Information at the end of this video.
[James]
I’m James. I’m a husband to a wonderful wife, father to three amazing kids. I’m a carpenter and woodworker, avid reader, always on a constant quest for knowledge. I’m a survivor, but most of all I’m a fighter, and this is my aTTP experience.
Back in 2015, I was, you know, delivering furniture for a company, started to feel a little bit of pain in my right abdomen, didn’t really think about it. As we started doing the first few deliveries, that pain intensified and, you know, my wife, she got to the point where, okay, you need to go to the hospital find out what’s going on. So, went to the hospital, was sent home, said they couldn’t find anything wrong with me. They really couldn’t explain the pain at that point. That feeling of dread, really, because I had no idea what was going on. So went home, and then ended up back in the ER and this point started noticing that, you know, my eyes became yellow, and my platelet level had dropped significantly. Hematologist ends up coming in, she had sent out for this one test, and it was a ADAMTS13 test. That’s when I was diagnosed with aTTP, and of course I never heard of it. I had no clue what I was dealing with, what was going on, and that’s when it became a battle.
[Dr Masias]
I am Dr Masias, and I have years of experience treating patients with aTTP. aTTP is a rare, potentially life-threatening disorder, which is why it’s so important to diagnose it early and treat it right away. Platelets are important components of blood that help stop bleeding when injury occurs. One of the ways the body can regulate platelet activity indirectly is via a protein called ADAMTS13 and von Willebrand factor. Normally, ADAMTS13 helps cut up von Willebrand factor. When you have aTTP, your immune system makes antibodies that attack ADAMTS13, preventing it from cutting the von Willebrand factor strands. When von Willebrand factor strands get too long, lots of platelets clump around them and those platelets can become blood clots that become stuck in the tiny blood vessels in the body. These clots can block the flow of blood to important organs such as the brain, heart, or kidneys. And this can cause serious health problems such as organ damage, stroke, or a heart attack.
It’s important to know what symptoms to look for. Low platelets can lead to bleeding from your gums and nose, bruises, petechiae, which are red dots that we see on the legs, and blood in the urine. Other symptoms that are common are chest pain, abdominal pain, confusion, tiredness, fatigue, and jaundice. These are some of the symptoms that I look for when I diagnose a patient with aTTP.
[James]
In 2019 my platelets dropped again and I had to go back into the hospital and this is when I was introduced to CABLIVI. It’s the first time I’ve heard of anything specific for aTTP. It lets me know that somebody is trying to work on this and trying to figure out how to help.
*CABLIVI was tested in a clinical study of 145 adults with aTTP/iTTP. 72 people were given CABLIVI, plasma exchange (PEX), and immunosuppressive therapy vs 73 people who were given placebo (an injection without any medicine), PEX, and immunosuppressive therapy. Both groups received either CABLIVI or placebo after their daily PEX, and then for 30 days after. Some people received more treatment (up to 28 additional days) based on their doctor’s decision. After treatment was stopped, everyone was followed by their doctor for 28 more days.*
[Dr Masias]
There are a few components when treating patients with aTTP with CABLIVI. Plasma exchange, also called plasmapheresis, is a process in which we take what a patient has a lot of, in this case antibodies against ADAMTS13, and it’s gonna also help us give the patient what they have little of, in this case ADAMTS13. Immunosuppression is going to help us control your immune system that is overactive when you have an episode of aTTP. CABLIVI, when used in combination with plasma exchange and immunosuppressive therapy, is gonna help prevent the formation of these very dangerous blood clots by preventing the interaction between von Willebrand factor and platelets.
I always talk to my patients about the potential side effects of CABLIVI. I believe in adding CABLIVI as soon as possible to all suitable adult patients with aTTP.
[James]
My platelet level went up when I had a CABLIVI in combination with my PEX and my immunosuppressant therapy. I didn’t experience any relapses while taking CABLIVI or for 28 days after. My doctor continues to monitor my blood work.
*Significantly fewer people had another episode of aTTP/iTTP that required starting PEX again within the full study period (while taking CABLIVI and 28 days after stopping CABLIVI) in the CABLIVI group vs the placebo group: 13% receiving CABLIVI (9 people) vs 38% receiving placebo (28 people). Individual results may vary.*
[Dr Masias]
I can’t stress enough the importance of a patient being educated on their disease, and I think it’s important for patients to know if these symptoms are related to their disease or this is something else that we need to diagnose and treat separately. By providing patients with education and being available to them, I think they can improve so much. It’s important that you discuss with your doctor if you think you’re a candidate for this medication.
[James]
Coming across CABLIVI was huge to me. My purpose to exist is to help educate others about aTTP. My oldest daughter, you know, she looked at me, you know, the one time and said you know you’re fighting this and you’re not giving up, you’re like you’re taking it on, you really are, you know, my superhero. That my kids see me like that is awesome to me. It really, it really inspires me. That’s my biggest goal to help whoever I can, however I can as far as aTTP.
This was James’s personal experience with CABLIVI. Every patient’s experience is unique and individual results will vary. If you’re interested in learning more, please speak with your doctor about the potential benefits and risks of treatment with CABLIVI and whether CABLIVI may be right for you.
INDICATION AND IMPORTANT SAFETY INFORMATION
What is CABLIVI?
CABLIVI (caplacizumab-yhdp) is a prescription medicine used for the treatment of adults with acquired thrombotic thrombocytopenic purpura (aTTP), in combination with plasma exchange and immunosuppressive therapy.
Who should not take CABLIVI?
Do not take CABLIVI if you’ve had an allergic reaction to caplacizumab-yhdp or to any of the ingredients in CABLIVI.
What should I tell my healthcare team before starting CABLIVI?
Tell your doctor if you have a medical condition including if you have a bleeding disorder. Tell your doctor about any medicines you take, including medicines that increase your risk of bleeding such as anti-coagulants and anti-platelet agents.
Talk to your doctor before scheduling any surgery, medical or dental procedure.
What are the possible side effects of CABLIVI?
CABLIVI can cause severe bleeding. In clinical studies, severe bleeding adverse reactions of nosebleed, bleeding from the gums, bleeding in the stomach or intestines, and bleeding from the uterus were each reported in 1% of subjects. In the post-marketing setting, cases of life-threatening and fatal bleeding were reported in patients receiving CABLIVI. Contact your doctor immediately if symptoms of excessive bruising, excessive bleeding, or major bleeding occur. Signs and symptoms of bleeding include: pain, swelling or discomfort, prolonged bleeding from cuts, increased menstrual flow or vaginal bleeding, nosebleeds, bleeding of gums from brushing, unusual bleeding or bruising, red or dark brown urine, red or tar black stools, headache, dizziness, or weakness.
You may have a higher risk of bleeding if you have a bleeding disorder (i.e. hemophilia) or if you take other medicines that increase your risk of bleeding such as anti-coagulants and anti-platelet agents.
CABLIVI should be stopped for 7 days before surgery or any medical or dental procedure. Talk to your doctor before you stop taking CABLIVI.
The most common side effects include nosebleed, headache and bleeding gums.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of CABLIVI. Call your doctor for medical advice about side effects.
CABLIVI, in combination with PEX and immunosuppression, can help you take on aTTP/iTTP with confidence
aTTP/iTTP=acquired/immune-mediated thrombotic thrombocytopenic purpura; TTP=thrombotic thrombocytopenic purpura.
INDICATION AND IMPORTANT SAFETY INFORMATION
Who should not take CABLIVI?
Do not take CABLIVI if you’ve had an allergic reaction to caplacizumab-yhdp or to any of the ingredients in CABLIVI.
What should I tell my healthcare team before starting CABLIVI?
Tell your doctor if you have a medical condition including if you have a bleeding disorder. Tell your doctor about any medicines you take, including medicines that increase your risk of bleeding such as antiplatelets, thrombolytics, heparin, or anti-coagulants.
Talk to your doctor before scheduling any surgery, medical or dental procedure.
What are the possible side effects of CABLIVI?
CABLIVI can cause severe bleeding. In clinical studies, severe bleeding adverse reactions of nosebleed, bleeding from the gums, bleeding in the stomach or intestines, and bleeding from the uterus were each reported in 1% of subjects. In the post-marketing setting, cases of life-threatening and fatal bleeding were reported in patients receiving CABLIVI. Contact your doctor immediately if symptoms of excessive bruising, excessive bleeding, or major bleeding occur. Signs and symptoms of bleeding include: pain, swelling or discomfort, prolonged bleeding from cuts, increased menstrual flow or vaginal bleeding, nosebleeds, bleeding of gums from brushing, unusual bleeding or bruising, red or dark brown urine, red or tar black stools, headache, dizziness, or weakness.
You may have a higher risk of bleeding if you have a bleeding disorder (i.e. hemophilia) or if you take other medicines that increase your risk of bleeding such as anti-coagulants and anti-platelet agents.
CABLIVI should be stopped for 7 days before surgery or any medical or dental procedure. Talk to your doctor before you stop taking CABLIVI.
The most common side effects in adults include nosebleed, headache and bleeding gums.
In children, the most reported side effects include nosebleed and rapid heartbeat.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of CABLIVI. Call your doctor for medical advice about side effects.
What is CABLIVI?
CABLIVI (caplacizumab-yhdp) is a prescription medicine used for the treatment of adults and children 12 years and older with acquired thrombotic thrombocytopenic purpura (aTTP), in combination with plasma exchange and immunosuppressive therapy.
Please see full Prescribing Information.
Instructions For Use
Sharps Medical Waste Disposal (PDF)
Learn more about Sanofi’s commitment to fighting counterfeit drugs.
INDICATION AND IMPORTANT SAFETY INFORMATION
Who should not take CABLIVI?
Do not take CABLIVI if you’ve had an allergic reaction to caplacizumab-yhdp or to any of the ingredients in CABLIVI.
What should I tell my healthcare team before starting CABLIVI?
Tell your doctor if you have a medical condition including if you have a bleeding disorder. Tell your doctor about any medicines you take, including medicines that increase your risk of bleeding such as antiplatelets, thrombolytics, heparin, or anti-coagulants.
Talk to your doctor before scheduling any surgery, medical or dental procedure.
What are the possible side effects of CABLIVI?
CABLIVI can cause severe bleeding. In clinical studies, severe bleeding adverse reactions of nosebleed, bleeding from the gums, bleeding in the stomach or intestines, and bleeding from the uterus were each reported in 1% of subjects. In the post-marketing setting, cases of life-threatening and fatal bleeding were reported in patients receiving CABLIVI. Contact your doctor immediately if symptoms of excessive bruising, excessive bleeding, or major bleeding occur. Signs and symptoms of bleeding include: pain, swelling or discomfort, prolonged bleeding from cuts, increased menstrual flow or vaginal bleeding, nosebleeds, bleeding of gums from brushing, unusual bleeding or bruising, red or dark brown urine, red or tar black stools, headache, dizziness, or weakness.
You may have a higher risk of bleeding if you have a bleeding disorder (i.e. hemophilia) or if you take other medicines that increase your risk of bleeding such as anti-coagulants and anti-platelet agents.
CABLIVI should be stopped for 7 days before surgery or any medical or dental procedure. Talk to your doctor before you stop taking CABLIVI.
The most common side effects in adults include nosebleed, headache and bleeding gums.
In children, the most reported side effects include nosebleed and rapid heartbeat.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of CABLIVI. Call your doctor for medical advice about side effects.
What is CABLIVI?
CABLIVI (caplacizumab-yhdp) is a prescription medicine used for the treatment of adults and children 12 years and older with acquired thrombotic thrombocytopenic purpura (aTTP), in combination with plasma exchange and immunosuppressive therapy.
Please see full Prescribing Information.
Instructions For Use
Sharps Medical Waste Disposal (PDF)
Learn more about Sanofi’s commitment to fighting counterfeit drugs.
INDICATION AND IMPORTANT SAFETY INFORMATION
Who should not take CABLIVI?
Do not take CABLIVI if you’ve had an allergic reaction to caplacizumab-yhdp or to any of the ingredients in CABLIVI.
What should I tell my healthcare team before starting CABLIVI?
Tell your doctor if you have a medical condition including if you have a bleeding disorder. Tell your doctor about any medicines you take, including medicines that increase your risk of bleeding such as antiplatelets, thrombolytics, heparin, or anti-coagulants.
Talk to your doctor before scheduling any surgery, medical or dental procedure.
What are the possible side effects of CABLIVI?
CABLIVI can cause severe bleeding. In clinical studies, severe bleeding adverse reactions of nosebleed, bleeding from the gums, bleeding in the stomach or intestines, and bleeding from the uterus were each reported in 1% of subjects. In the post-marketing setting, cases of life-threatening and fatal bleeding were reported in patients receiving CABLIVI. Contact your doctor immediately if symptoms of excessive bruising, excessive bleeding, or major bleeding occur. Signs and symptoms of bleeding include: pain, swelling or discomfort, prolonged bleeding from cuts, increased menstrual flow or vaginal bleeding, nosebleeds, bleeding of gums from brushing, unusual bleeding or bruising, red or dark brown urine, red or tar black stools, headache, dizziness, or weakness.
You may have a higher risk of bleeding if you have a bleeding disorder (i.e. hemophilia) or if you take other medicines that increase your risk of bleeding such as anti-coagulants and anti-platelet agents.
CABLIVI should be stopped for 7 days before surgery or any medical or dental procedure. Talk to your doctor before you stop taking CABLIVI.
The most common side effects in adults include nosebleed, headache and bleeding gums.
In children, the most reported side effects include nosebleed and rapid heartbeat.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of CABLIVI. Call your doctor for medical advice about side effects.
What is CABLIVI?
CABLIVI (caplacizumab-yhdp) is a prescription medicine used for the treatment of adults and children 12 years and older with acquired thrombotic thrombocytopenic purpura (aTTP), in combination with plasma exchange and immunosuppressive therapy.
Please see full Prescribing Information.
Instructions For Use
Sharps Medical Waste Disposal (PDF)
Learn more about Sanofi’s commitment to fighting counterfeit drugs.



